Uses of wrought iron
Wrought iron
manufacturing process of wrought iron
. properties and uses of wrought iron
Wrought iron is the purest form of iron. The analysis of wrought iron shows as much as 99.9% of iron.
When heated, wrought iron does not melt, but only becomes pasty and in this form it can be forged to any shape.
Modern methods used to produce wrought iron in large quantities are the
-pudding process
-aston or byers process
Pudding process
Wrought iron is manufactured by refining pig-iron.
By refining pig-iron silicon is removed completely, a greater amount of phosphorus is removed, and graphite is converted to combined carbon.
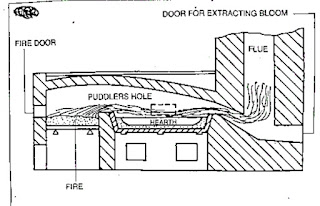
The above process is carried out in a pudding furnace.
Pudding furnace
This furnace is a coal-fired reverberatory furnace.
The term reverberatory is applied because the charge is not in actual contact with the fire, but receives its heat by reflection from the dome shaped furnace roof.
The product obtained is taken out from the furnace in the form of balls (or blooms) having a mass of about 50kgs.
The hot metal is then passed through grooved rollers which convert blooms into bars called MUCK BARS or PUDDLE BARS.
These bars are cut into short lengths, fastened together in pilers, reheated to welding temparature and again rolled into bars.
Aston process
In this process molten pig iron and steel scrap are refined in a bessemer converter.
The refined molten metal is poured into an open hearth furnace in the iron silicate stage. This removes most of the carbon.
The slag cools the molten metal to a pasty mass which is later squeezed in a hydraulic press to remove most of the slag. Rectangular blocks known as blooms are formed from this mass.
The hot bloom is immediately passed through rolling mills to produce products of wrought iron of different shapes and sizes.
COMPOSITION OF WROUGHT IRON
Carbon - 0.02 to 0.03%
Silicon - 0.1 to 0.2%
Manganese - 0.02 to 0.1%
Sulphur - 0.02 to 0.04%
Phosphorus - 0.05 to 0.2%
Iron forms of the rest of the content.
PROPERTIES AND USES OF WROUGHT IRON
PROPERTIES
Malleable and ductile. It can neither be hardened not tempered. Tough, shock resistant fibrous structure easy for forge welding. Ultimate tensile strength of about 350 newtons per sq. mm. No effect in salt water. Will not retain the magnetism. Corrosion resistant. Easy to forge wide temparature range 850° c to 1350° c.
USES
Architectural works.
Crane hooks, chain links, bolts and nuts and railway coupling.
Marine works.
Temporary magnets. Core of dynamos.
Agricultural equipment.
Pipes, flanges etc.
SURFACE PREPARATION
Surface carrying dirt, grease, corrosion or mill scale are unsuitable for the direct application of anti corrosion treatment.
The most important factor for any efficient anti corrosion treatment is surface preparation.
The different methods used for surface preparation are:
--degreasing
-pickling
-blast cleaning
-flame descaling
Degreasing
In degreasing, the surface preparation for anti corrosion treatment is done with a solvent such as:
-white spirits
-carbon tetrachloride
-trichlorethylene
The solvents used in degreasing will create a health hazard. Safety precaution should be taken before using these solvents.
Pickling
Pickling is a chemical method of cleaning.
The surface of the metal is cleaned with dilute sulphuric acid or mixed acids.
Blast cleaning
This is a mechanical method of a cleaning in which the scale and corrosion are removed by a high velocity blast of steel shots or sand particles.If steel shorts are used it is called sand blasting.
Flame descaling
The steel surface which is to be descaled, is heated with an oxy-fuel gas torch with high intensity flames.
This rapid local thermal expansion of the loosely adhering scale against the relatively un heated base metal causes the scale to flake off.
This process is excellent for removing rust. Any rust particles converted to powder can easily be brushed away before commencing to paint. For best results the primer should be applied when the steel is approximately 45°c, that is the temparature at which the hand can hold the steel comfortably.
This method of surface preparation is often specified for the heavy rusted steel work. It is not suitable for light steel work which may buckle and distort because of the intense localized that.






Comments
Post a Comment